Thermal phenomena and thermal runaway
It has been established that during operation of the Closed Oxygen Cycle (COC) on constant voltage polarization, the cell reaches a state where the reactions of the two electrodes get into self-accelerating interrelation. Both cell current and temperature begin to increase spontaneously and pass through a maximum before reaching stationary values. This phenomenon is called thermal run away (TRA). The processes that initiate thermal phenomena and may cause thermal runaway that could lead to failure of VRLA batteries have been investigated in our laboratory. Through following the changes in U, I, T, j-, j+, Vgas (H2) of VRLA cells polarized at high voltages a model for the processes causing TRA has been proposed.
Stages of thermal runaway phenomena. Progress of TRA could be divided in four different stages.
- A-stage. H2 and O2 are evolved on the plates. O2 passes through the AGM separator and is reduced on the negative plate. The potential of the negative electrode decreases, while that of the positive one increases.
- B-stage. Reduction of O2 proceeds with generation of heat, which leads to temperature rise. At high temperatures O2 overvoltage decreases, I increases, the O2 flow through AGM and its reduction rate increase, too. More heat is evolved, T rises rapidly. A loop of Self-Accelerating InterRelations between the reactions of the oxygen cycle on the two Electrodes (SAIRE) is formed. Since the temperature rises the mechanism of oxygen reduction changes from electrochemical to chemical one. The chemical mechanism involves exothermic chemical reactions.
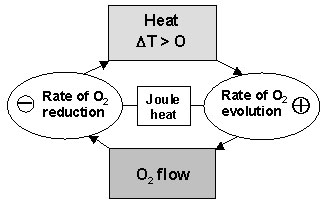
- C-stage. The above processes are associated with consumption or production of water. The H4SO4 concentration at the positive plate increases, whereas that at negative plate decreases. These concentration changes lead to changes in structure and physico-chemical properties of the two types of plates, which influences the reactions that proceed there. The higher H4SO4 concentration causes passivation of the positive electrode. The cell current reaches a maximum value and decreases thereafter.
- D-stage. The cell temperature reaches a maximum and starts to decrease, which is related to dissipation of heat to the surrounding medium.
To avoid the appearance of TRA phenomena, an AGM separator with appropriate physico-chemical properties should be selected, the current and voltage should be kept below certain critical values.
References
- D. Pavlov, Energy balance of the closed oxygen cycle and processes causing thermal runaway in valve-regulated lead/acid batteries, J. Power Sources, 64 (1996) 131
- D. Pavlov, B. Monahov, A. Kirchev, D. Valkovska, Thermal runaway in VRLAB – Phenomena, reaction mechanisms and monitoring, J. Power Sources, 158 (2006) 689
- D. Pavlov, Thermal phenomena during operation of the oxygen cycle in VRLAB and processes that cause them, J. Power Sources, 158 (2006) 964
Keywords: thermal runaway, failure of VRLA batteries, heat generation in VRLAB, Self-Accelerating InterRelations between COC reactions, exothermic chemical reactions in VRLAB, PbO2 electrode passivation

