Electrochemistry of antimony electrode in H2SO4 solution
Antimony (Sb) is widely used as alloying additive to lead alloys for casting lead-acid battery grids. It has been established that it affects significantly not only the mechanical properties of the alloys but also has strong impact on the electrochemical properties of positive battery plates. The electrochemical behavior of Sb electrode in sulphuric acid solution has been investigated at LABD.
It has been established that anodic polarization of antimony electrode in H2SO4 solutions could be divided in three potential regions.
α-Region. This region is between –0.47 and –0.39 V vs. Hg/Hg2SO4 reference electrode. Within this potential region, active dissolution of Sb proceeds. The reaction steps in the corrosion process and the mechanism of the elementary processes have been identified.
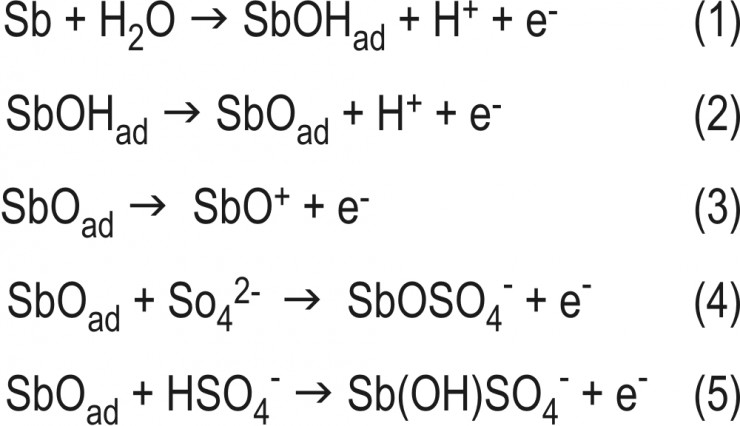
ß-Region. In this region of relatively low overpotentials, a surface film is formed on the metal surface and starts to passivate the electrode. Through SEM observations and X-ray diffraction analysis the very thin and amorphous gel-like passive layer has been analyzed.
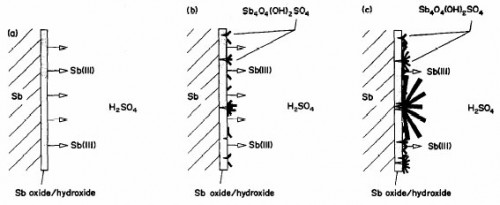
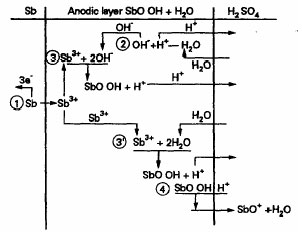
The processes that take place in the passive layer have been elucidated. A reaction scheme has been proposed for the formation and dissolution of the primary anodic layer during oxidation of Sb in H2SO4.
γ-Region. In this region of high anodic overpotentials, passive dissolution of antimony proceeds. At high overpotentials conditions for formation of a new layer with barrier properties are created. The kinetics of growth of the barrier layer is limited by high-field assisted ion migration. Formation of the barrier layer facilitates nucleation and growth of a thermodynamically stable phase (klebelsbergite).
References
- D. Pavlov, M. Bojinov, T. Laitinen, G. Sundholm, Electrochemical Behaviour of the Antimony Electrode in H2SO4 Solutions. I. Corrosion and Active Dissolution of Antimony, Electrochim. Acta, 36 (1991), 2081
- D. Pavlov, M. Bojinov, T. Laitinen, G. Sundholm, Electrochemical Behaviour of the Antimony Electrode in H2SO4 Solutions. II. Formation and Properties of the Primary Anodic Layer, Electrochim. Acta, 36 (1991) 2087
- T. Laitinen, H. Revitzer, G. Sundholm, J. Vilhunen, D. Pavlov, M. Bojinov, Electrochemical Behaviour of the Antimony Electrode in H2SO4 Solutions. III. Identification of Corrosion Products after Long-Term Polarization, Electrochim. Acta, 36 (1991) 2093
- M. Bojinov, D. Pavlov, The Processes of Formation of a Gel-Like Anodic Layer During Polarization of an Antimony Electrode in H2SO4 Solution, J. Electroanal. Chem., 315 (1991) 201
- M. Bojinov, D. Pavlov, Anodic Oxidation of Antimony at High Anodic Overpotential - Formation of a Barrier Layer and Klebelsbergite, J. Electroanal. Chem., 346 (1993) 339
- M. Bojinov, D. Pavlov, The Antimony/Klebelsbergite Electrode, J. Electroanal. Chem., 367 (1994) 195
Keywords: antimony electrode in sulphuric acid solution, dissolution of Sb, passivation of antimony electrode, dissolution of Sb passive film


