Microstructure, chemical and electrochemical properties of PbO2 in the positive active mass
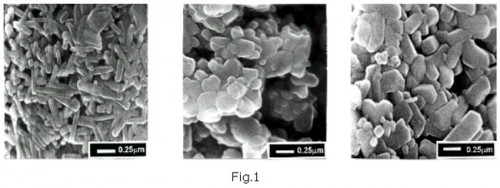
Structure of PbO2 particles. Figure 1 presents three types of PbO2 particles: spherical or egg-shaped, PbO2 crystal particles and needle-like particles.
A heterogeneous mass distribution is observed in the bulk of PbO2 particles (Fig.2).

Dark zones have crystal structure ( α or β PbO2) and size 20 to 40 nm. More electron transparent zones are hydrated (gel zones). Hence, PbO2 particles have crystal/gel structure. About 31-34% of PAM is hydrated.
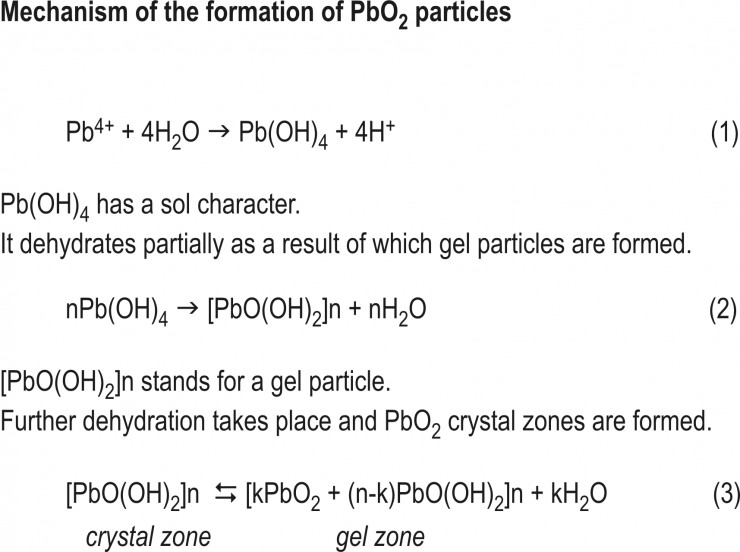
Hydrated zones exchange ions with the solution (PbO2 particle is an open system). The ratio between crystal and gel zones influences the capacity of the plate.
The processes that occur at the interfaces particle/solution and crystal zones/gel zones are almost reversible and relatively fast. These phenomena allow electrochemical reactions with low polarization to proceed in the bulk of agglomerates during discharge of positive plates. Crystal PbO2 is a degenerated n-type semi-conductor, which is responsible for the high electron conductivity of PAM. Amorphous gel zones can be represented as built of linear polymer hydrated chains. They determine the proton and electron conductivity of PAM.
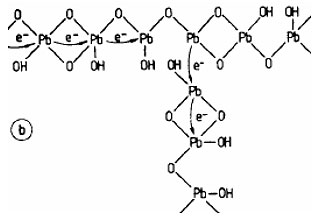
An “island – bridge” mechanism of electron movement between crystal and gel zones is proposed.
Electrochemical activity of PbO2 particles. The concurrent existence of crystal and gel zones in the agglomerates determine the electrochemical activity of lead-acid battery positive plates. In view of the gel-crystal structure of PAM this activity should be influenced by the ratio between crystal and gel zones, the density of PAM and the amount and type of dopands used. It has been found that the phase composition of PAM depends on pH of the solution since the hydrated parts of PbO2 particles interact with ions in the solution as a result of which the crystal zones/hydrated zones and hydrated zones/solution equilibriums change. Since solution pH influences the phase composition of PAM it affects the electrochemical performance of PbO2 . The effect of H2SO4 concentration on the mechanisms of the processes of reduction of PbO2 and oxidation of the formed PbSO4 has been elucidated and three concentration regions have been identified. The phase composition and crystal morphology of corrosion layer and PAM have been examined.
References
- D. Pavlov, E. Bashtavelova, V. Manev, A. Nasalevska, Effect of chemisorbed water on the electrical capacity of the lead-acid battery positive plate, J. Power Sources, 19(1987) 15
- D. Pavlov, Reply to Comments of R.J. Hill on "Effect of chemisorbed water on the electrical capacity of lead-acid battery positive plate", J. Power Sources, 22 (1988) 179
- D. Pavlov, I. Balkanov, T. Halachev, P. Rachev, Hydration and amorphization of active mass PbO2 particles and their influence on the electrical properties of the lead-acid battery positive plate, J. Electrochem. Soc., 136 (1989) 3189
- D. Pavlov, PbO2 Particles of the Positive Active Mass - A Complex Open Electrochemical System, Extended Abstracts, The 30th Battery Symposium in Japan, Nagoya, 1989, p.3
- D. Pavlov, I. Balkanov, The PbO2 Particle: Exchange Reactions Between Ions of the Electrolyte and the PbO2 Particles of the Lead-Acid Battery Positive Active Mass, J. Electrochem. Soc., 139 (1992) 1830
- D. Pavlov, The Lead-Acid Battery Lead Dioxide Active Mass - a Gel-Crystal System with Proton and Electron Conductivity, J. Electrochem. Soc., 139 (1992) 3075
- D. Pavlov, Influence of Crystal and Gel Zones on the Capacity of the Lead Dioxide Active Mass of Lead-Acid Battery, J. Power Sources, 40 (1992) 169
- M. Dimitrov, D. Pavlov, Influence of grid alloy and fast charge on battery cycle life and structure of the positive active mass of lead acid batteries, J. Power Sources, 93 (2001) 234
- B. Monahov, D. Pavlov, A. Kirchev and S. Vasilev, Influence of pH of the H2SO4 solution on the phase composition of the PbO2 active mass and of the PbO2 anodic layer formed during cycling of lead electrodes, J. Power Sources, 113 (2003) 281
- D.Pavlov, A. Kirchev, M. Stoycheva, B. Monahov, Influence of H2SO4 concentration on the mechanism of the processes and on the electrochemical activity of the Pb/PbO2/PbSO4 electrode, J. Power Sources, 137 (2004) 288
Keywords: electrochemical activity of lead dioxide, PbO2 particles structure, crystal/gel zones model of PbO2 , microstructure of PAM, PbO(OH)2 structure


