Electrochemical systems on polarization of lead electrodes
Earliest research works and papers of LABD were devoted to investigations into the anodic behavior of the lead electrode. When Pb electrode is immersed in sulphuric acid solution and polarized at oxidation potentials, a dense crystalline layer forms on the electrode surface and the latter gets passivated. By varying the oxidation potential and employing XRD analysis and SEM observations D. Pavlov and coworkers determined the phase composition and morphological structure of the anodic layer as a function of applied potential.
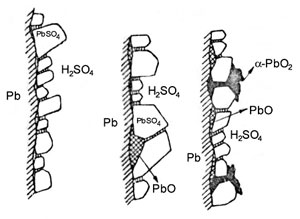
Three different potential regions were distinguished:
- Lead sulphate region: on polarization between –0.95 and –0.40 V vs. Hg/Hg2SO4 reference electrode. The anodic layer is composed of PbSO4 crystals, which form the Pb/PbSO4/H2SO4 electrode system.
- Lead oxide region: on polarization between -0.40 and +0.95 V. The anodic deposit consists of PbSO4 and tet–PbO sub-layers. They form the Pb/PbO/PbSO4/H2SO4 electrode system.
- Lead dioxide region: on polarization above +0.95 V the basic components of the anodic layer formed are α-PbO2 and ß-PbO2.
Following this fundamental study on the formation of the above electrochemical systems further in-depth theoretical and experimental research was conducted aimed to elucidate the physico-chemical and electrochemical characteristics of each of the above electrode systems, and its influence on lead-acid battery performance. Kinetic models and reaction mechanisms were proposed for each electrode system and the influence of various parameters was investigated.
References
- D. Pavlov, Mechanism of the anodic oxidation of lead in sulphuric acid solution, Berichte der Bunsen-geselschaft, 71 (1967) 398
- D. Pavlov, C.N. Poulieff, E. Klaja, N. Iordanov, Dependence of the composition of anodic layer on the oxidation potential of lead in sulphuric acid, J. Electrochem. Soc., 116 (1969) 316
- D. Pavlov, N. Iordanov, Growth processes of the anodic crystalline layer on potentiostatic oxidation of lead in sulphuric acid, J.Electrochem. Soc., 117 (1970) 1103
- D. Pavlov, Mechanism of the Processes during anodic oxidation of a lead electrode in sulphuric acid solutions, Advances in Lead-Acid Batteries, Proceedings of the International Symposium, Vol. 84-14, p. 110, Electrochem. Soc. Inc., Pennington, USA, 1984
Keywords: lead electrodes, Pb/H2SO4 electrode, passivation, lead electrode systems, lead sulphate potential region, Pb/PbSO4/H2SO4 electrode, lead oxide potential region, Pb/PbO/PbSO4/H2SO4 electrode, lead dioxide potential region

