Formation of lead sulphate membrane and passivation of the lead electrode
It has been established, through scanning electron microscopy, that the PbSO4 layer formed on anodic polarization of Pb electrode in H2SO4 solution has membrane properties. This PbSO4 layer screens the electrode surface and the electrochemical reaction proceeds only on that part of the surface which is free of PbSO4 crystals. Lead ions pass through the pores of the PbSO4 layer. Depending on the size of these pores two types of electrode passivation are observed.
Thermodynamic passivation. Pavlov proposed the following mechanism. The PbSO4 layer acts as a semi-permeable membrane that allows H2O, H+, OH- and Pb2+ ions to pass through but stops the large SO42- and HSO4- ions. In order to preserve the solution in the pores electroneutral, the latter is alkalized. The electrode potential on open circuit is between -750 and -650 mV, and it does not change with time. P. Ruetschi measured experimentally the potential of the membrane electrode and confirmed Pavlov's mechanism, which is now known as "the mechanism of Pavlov-Ruetschi".
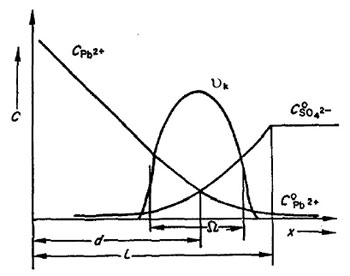
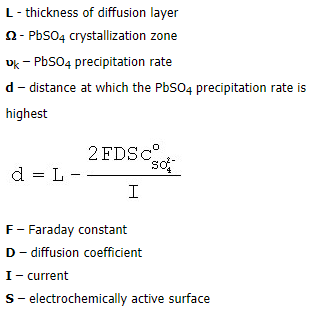
Kinetic passivation. The pores in the PbSO4 layer are larger than those in the semi-permeable membrane and hence all ions can move freely through the pores. When the flow of lead ions is larger than that of sulphate ions entering the PbSO4 pores, the reaction between them proceeds at the pore orifice near the electrolyte. To keep the solution in the pore interior neutral, it is alkalized and basic lead sulphate and/or lead oxide are formed. On opening of the circuit, the basic lead sulphate and PbO crystals react with H2SO4 as a result of which the pores are opened thus allowing access of H2SO4 to the lead surface. The electrode potential is equal to the equilibrium potential of the Pb/PbSO4 electrode, i.e. -950 mV.
References
- D. Pavlov, Processes of formation of divalent lead oxide compounds on anodic oxidation of lead in sulphuric acid, Electrochim. Acta, 13 (1968) 2051.
- D. Pavlov, R. Popova, Mechanism of passivation processes of the lead sulphate electrode, Electrochim. Acta, 15 (1970) 1483.
- D. Pavlov, B. Monakhov, Effect of Sb on the electrochemical properties of Pb/PbSO4/H2SO4 and Pb/PbO/PbSO4/H2SO4 electrodes, J. Electroanal. Chem., 218 (1987) 135.
- T. Laitinen, B. Monahov, K. Salmi, G. Sundholm, Ring-disk electrode studies of soluble intermediates formed during the polarization of Pb in H2SO4, Electrochimica Acta, 36(1991) 953.
DSc Thesis
D. Pavlov, "Mechanism of the processes on anodic oxidation of lead in sulphuric acid solution"
Keywords: PbSO4 semi-permeable membrane, thermodynamic passivation, pore solution alkalization mechanism, basic lead sulphates, formation of lead oxide, kinetic passivation