Mechanism of the processes during charge and discharge of positive battery plate
The overall reactions of discharge and charge can be represented by the following equation:
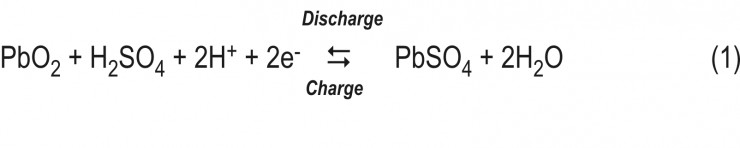
These are the final products of the reactions. The mechanism of the elementary reactions and processes depends on the structure of PAM, the pH of the pore solution and the ion transport through the pores. It involves the formation of a number of intermediate products.
Processes during discharge. It has been found through micro-diffraction analysis that parallel to PbSO4 orthorhombic PbO is also formed during discharge of the positive plate [1]. The discharge reaction proceeds in the hydrated gel zones of a certain number of PbO2 particles and aggregates (active centres). A scheme of the reactions that proceed in the active centres is presented in Fig. 1.
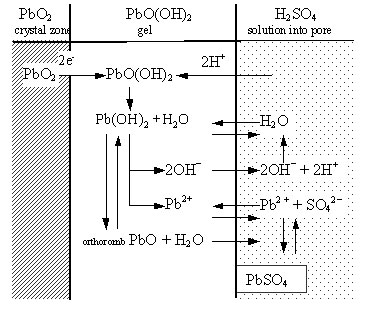
Electrons pass from the crystal zones (PbO2) into the gel zones and H+ ions from the solution in the pores enter these gel zones (to preserve electroneutrality), whereby PbO(OH)2 is reduced to Pb(OH)2. When a given gel zone is in contact with H2SO4 solution, a reaction of PbSO4 formation proceeds. In the aggregates interior access of H2SO4 to the micropores is impeded and Pb(OH)2 is partially dehydrated to orthorhomb-PbO [1-3]. Gel zones are in equilibrium with crystal zones and with ions in the solution. A reaction of hydration PbO2 + H2O → PbO(OH)2 proceeds, which supports the existence of the gel zones and thus the discharge reaction until the whole PbO2 particle or agglomerate is reduced. On further discharge and on open circuit Pb2+ ions released by Pb(OH)2 diffuse from the micropores in the aggregates’ interior to the solution in the macropores. These ions contribute to the formation and growth of PbSO4 crystals in the macropores [4, 5]. The latter process has been confirmed also by measuring the changes in PAM real density and total pore volume during discharge [6]. The density of the phases formed during discharge is higher than the value corresponding to the amount of PbSO4 as calculated using equation (1) and Faraday’s law. This is related to the formation of PbO. Only after a 16-hour stay on open circuit does the PAM density decrease, due to sulfation of PbO, to a value corresponding to the calculated PbSO4 quantity according to Faraday equation [6,7]. After that PbSO4 recrystallizes into larger crystals. The driving forces of PbSO4 recrystallization have been disclosed [8]. As a result of this process the positive active mass shrinks [9]. It has been established that at the beginning of cycling the plate capacity depends on the volume of transport pores and less so on the BET surface of PAM [9].
Processes during charge. The elementary reactions that proceed during plate charge are presented schematically in Fig. 2.
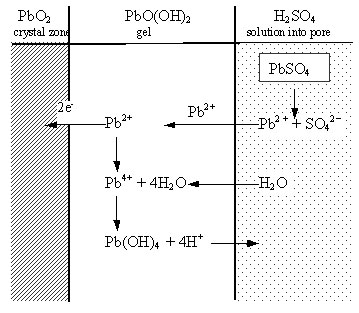
PbSO4 crystals maintain a certain concentration of Pb2+ ions in the pore solution. These are in equilibrium with the Pb2+ ions in the gel zones. When the plates are anodically polarized, electrons released by the Pb2+ ions in the gel zones enter the crystal PbO2 zones. The equilibrium between Pb2+ ions in the gel and in the solution is restored through transfer of Pb2+ ions from the solution into the gel zones. PbSO4 crystals dissolve to maintain the equilibrium concentration of Pb2+ ions in the solution filling the pores. On the other hand, water enters the gel zones and reacts with Pb4+ ions. H+ ions leave the gel zones to keep their electroneutrality. Groups of Pb(OH)4 molecules interconnect to form particles which are partially dehydrated. Thus the aggregates grow in size at the expense of oxidation of PbSO4 crystals, and new particles are formed. The particles interlock to form agglomerates.
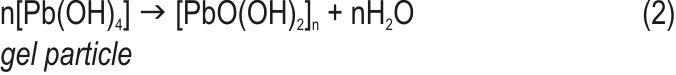
Dehydration of the particles continues until crystal zones are formed in the volume of particles and agglomerates.
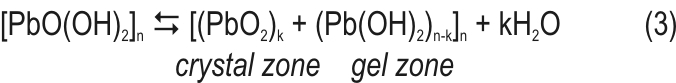
An equilibrium is established between crystal and gel zones [4] on the one hand, and between gel zones and the solution in the pores, on the other hand [12, 5]. The above processes occur at certain sites (active centres) in PAM, where the gel has a definite concentration and stoichiometry.
If the diffusion path of Pb2+ ions from the site of PbSO4 dissolution to the active centres is short, the obtained PbO2 agglomerates and aggregates preserve the shape of the PbSO4 crystal matrix and a lead dioxide aggregate is formed (metasomatic mechanism). If, however, this is a long way, PbO2 agglomerates “forget” the shape of the mother PbSO4 crystal (free formation of PbO2 aggregates and agglomerates) [10].
The charge and discharge processes on cycling cause changes in the structure of PAM. The molar volume of PbSO4 is larger than that of PbO2 . That is why the discharged plate is thicker than the charged one. On plate cycling, the PAM pulsates (“breathing”), increasing its volume and weight on discharge and decreasing (both in volume and weight) on charge [9]. As certain active centres in PAM are involved in the discharge processes and others in the charge reactions, the structure of PAM changes. This may lead to impaired contacts between the individual aggregates and agglomerates in PAM, and between PAM and the grid. As a result of this the capacity declines. It has been found that alloying additives such as Sb, As and Bi slow down the process of disintegration of PbO2 aggregates and thus support the integrity of the PAM skeleton, as a result of which the cycle life of the positive plates improves.
References
- D. Pavlov, I. Balkanov, P. Rachev, Orthorhombic PbO formation during discharge of lead-acid batteries PbO2 active mass, J. Electrochem. Soc., 134 (1987) 2390
- D. Pavlov, E. Bashtavelova, V. Iliev, Processes during the discharge of the positive lead-acid battery plate, 32 Meeting of ISE, Extended Abstracts, Part I, p.146, Dubrovnik, Yugoslavia, 1981
- D. Pavlov, I. Pashmakova, Influence of the size of PbSO4 crystals on their solubility and the significance of this process in the lead-acid battery, J. Appl. Electrochem., 17 (1987) 1075
- D. Pavlov, Processes during Discharge of the Lead-Acid Battery PbO2 Active Mass, Proceedings 31st International Congress IUPAC-87, p. 162, Sofia, Bulgaria, 1987
- D. Pavlov, Discharge processes in lead-acid battery positive plates, Power Sources - 11, 15th Intl. Symposium, Brighton 1986, Ed. L.J. Pearce, Pergamon Press, p. 165, London, 1987
- D. Pavlov, E. Bashtavelova, D. Simonsson, P. Ekdunge, Processes at the Micro-Level in the Oxidation of PbSO4 to PbO2 during Charging of Lead/Acid Battery Positive Plates, J. Power Sources, 30 (1990) 77
- M. Bojinov, D. Pavlov, Electrical and electrochemical properties of PbO2 plate of lead-acid cell, Proceedings of extended Abstracts, LABAT’96 International conference, p.83, Varna, June 1996
- G. Papazov, D. Pavlov, Influence of cycling current and power profiles on the cycle life of lead-acid batteries, Proceedings of extended Abstracts, LABAT’96 International conference, p.3, Varna, June 1996
- G. Papazov, D.Pavlov, Influence of cycling current and power profiles on the cycle life of lead-acid batteries, J. Power Sources, 62 (1996) 193
- M. Dimitrov, D. Pavlov, M. Shiomi, M. Tsubota, Influence of fast charge on the structure and properties of lead-dioxide active mass, Proceedings of International conference LABAT’99, Sofia, 7-10 June 1999, p.75
- G. Papazov, B. Monahov, D. Pavlov, Influence of PAM density on the cycle life of positive lead-acid battery plates, Proceedings of International conference LABAT’99, Sofia, 7-10 June 1999, p.65
- D. Pavlov, G. Petkova, M. Dimitrov, M. Shiomi, M. Tsubota, Influence of fast charge on the cycle life of positive lead-acid batteries plates, J. Power Sources, 87 (2000) 39
- D. Pavlov, G. Petkova, Phenomena that limit the capacity of the positive lead acid battery plates. 1. The charge potential transient as an indicator of positive plate state of charge and state of health. J. Electrochem. Soc., 149 (2002) A644
- D. Pavlov, G. Petkova, Phenomena that limit the capacity of the positive lead acid battery plates. 2. Electrochemical impedance spectroscopy and mechanism of discharge of the plate. J. Electrochem. Soc., 149 (2002) A654
- G. Petkova, D. Pavlov, Influence of charge mode on the capacity and cycle life of lead-acid battery negative plates, Proceedings of International Conference LABAT’02, Varna, 10-13 June 2002, p. 119
- G. Petkova and D. Pavlov, Influence of charge mode on the capacity and cycle life of lead–acid battery negative plates, J. Power Sources, 113 (2003) 355
- D. Pavlov, A. Kirchev, M. Stoycheva, B. Monahov, Influence of H2SO4 concentration on the mechanism of the processes and on the electrochemical activity of the Pb/PbO2/PbSO4 electrode, J. Power Sources, 137 (2004) 288
Keywords: PAM charge, PAM discharge, mechanism of the elementary reactions of PAM charge, mechanism of the elementary reactions of PAM discharge, metasomatic mechanism of PAM discharge

