Oxygen reduction at the negative plates
The LABD team has performed fundamental investigations into the mechanism of reactions and elementary processes involved in the closed oxygen cycle in VRLAB. The influence of temperature and active block saturation with electrolyte has been determined based on a newly proposed model for the reactions of oxygen reduction.
Mechanism of the reactions of oxygen reduction. A general mechanism of the reactions of oxygen reduction at the negative plate involved in the closed oxygen cycle (COC) in VRLAB has been proposed. Parallel reactions of oxygen reduction and hydrogen evolution proceed on the surface of the lead crystals of NAM. Oxygen reduction, which results in water generation, proceeds through an electrochemical mechanism of oxygen reactions and a chemical mechanism of reactions between the intermediate products of oxygen and hydrogen reactions at the negative plate. According to this mechanism, the reduction of O2 at the negative plate proceeds through formation of H2O2 first. Part of the H2O2 (strong oxidant) is electrochemically reduced to H2O (electrochemical mechanism). Another part of H2O2 reacts with H atoms (strong reducer) adsorbed on the surface of lead crystals forming H2O through chemical reaction (chemical mechanism). The chemical interaction between H2O2 and Pb leads to a series of exothermic reactions that result in formation of lead compounds and water. This is the other branch of reactions of the chemical mechanism of oxygen reduction.
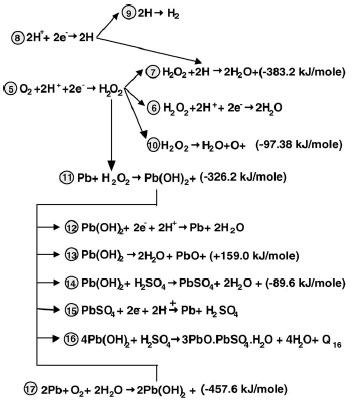
Both mechanisms are in competition since they use one and same initial substance – H2O2. Which of the two mechanisms will predominate depends on negative plate potential and saturation level, and on cell temperature. Both mechanisms proceed at high rates. At low temperatures the electrochemical mechanism is the predominating one, whereas at high temperatures chemical formation of water is predominating.
Elementary processes of electrochemical reduction of O2 It is assumed that oxygen diffusion through the thin liquid film (TLF) covering NAM and impeded charge transfer through the electric double layer are the rate limiting steps of the closed oxygen cycle (COC). A model for the oxygen recombination kinetics in VRLAB is proposed, which accounts for the processes that take place in the TLF covering the surface of NAM.
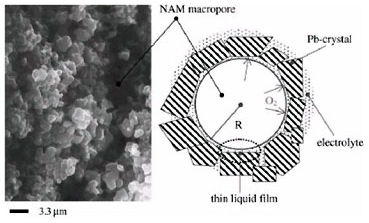
By employing the DLVO theory of colloid systems the thickness of TLF and its influence on COC has been estimated. A general equation has beens obtained for the relation between oxygen recombination rate and TLF properties, NAM structure and polarization time. Experimental methods have been developed in LABD for determining both the current of electrochemical reduction of oxygen and the current of oxygen cycle by measuring the gas flow leaving the cell during overcharge. The validity of the equation has been experimentally confirmed for different electrolyte saturation levels of plates, active block compressions and additives to NAM.
Influence of temperature and electrolyte saturation on oxygen cycle rate and efficiency in VRLAB. The influence of temperature and electrolyte saturation of the active block on the rate and efficiency of oxygen cycle in VRLAB has been estimated. Negligible temperature dependence of oxygen cycle rate has been found. Experimental results have shown that with increase of temperature the rate of H2 evolution increases thus reducing oxygen cycle efficiency. The O2 cycle current depends on AGM or NAM saturation with electrolyte. A decrease in saturation level results in more efficient oxygen cycle. The optimum conditions for efficient operation of the oxygen cycle are: temperature below 40oC; negative plate potential below –1.20 V vs. Hg/Hg2SO4; electrolyte saturation level about 93%
References
- D. Pavlov, S. Ruevski, V. Naidenov, G. Sheytanov, Influence of temperature, current and number of cycles on the efficiency of the closed oxygen cycle in VRLA batteries, J. Power Sources, 85 (2000) 164
- A. Kirchev, D. Pavlov and B. Monahov, Gas-diffusion approach to the kinetics of oxygen recombination in lead-acid batteries, J. Power Sources, 113 (2003) 245
- D. Pavlov, A. Kirchev, B. Monahov, Mechanism of the oxygen cycle reactions proceeding at the negative plates of VRLAB, J. Power Sources, 144 (2005) 521
- D. Pavlov, Thermal phenomena during operation of the oxygen cycle in VRLAB and processes that cause them, J. Power Sources, 158 (2006) 964
- A. Kirchev, D. Pavlov, Influence of temperature and electrolyte saturation on rate and efficiency of oxygen cycle in VRLAB, J. Power Sources, 162 (2006) 864
Theses
A. Kirchev, PhD: Gas-diffusion kinetics of oxygen reduction on the negative plate in valve regulated lead-acid batteries.
Keywords: closed oxygen cycle, oxygen reduction on NAM, electrochemical reduction of oxygen, chemical reduction of oxygen, oxygen recombination kinetics, thin liquid film, electrolyte saturation of VRLAB, temperature dependence of oxygen cycle, oxygen cycle efficiency

